
Over the past 3 years, drug manufacturers have disrupted the medical and prescription drug industries considerably with the rapid growth of genetic and cellular (gene and cell) therapies. Often lifesaving, these therapies represent a promising new era for medical treatments in the US. However, the high cost and growing pipeline for gene and cell therapies present considerable challenges for the US medical insurance market.
Current Landscape
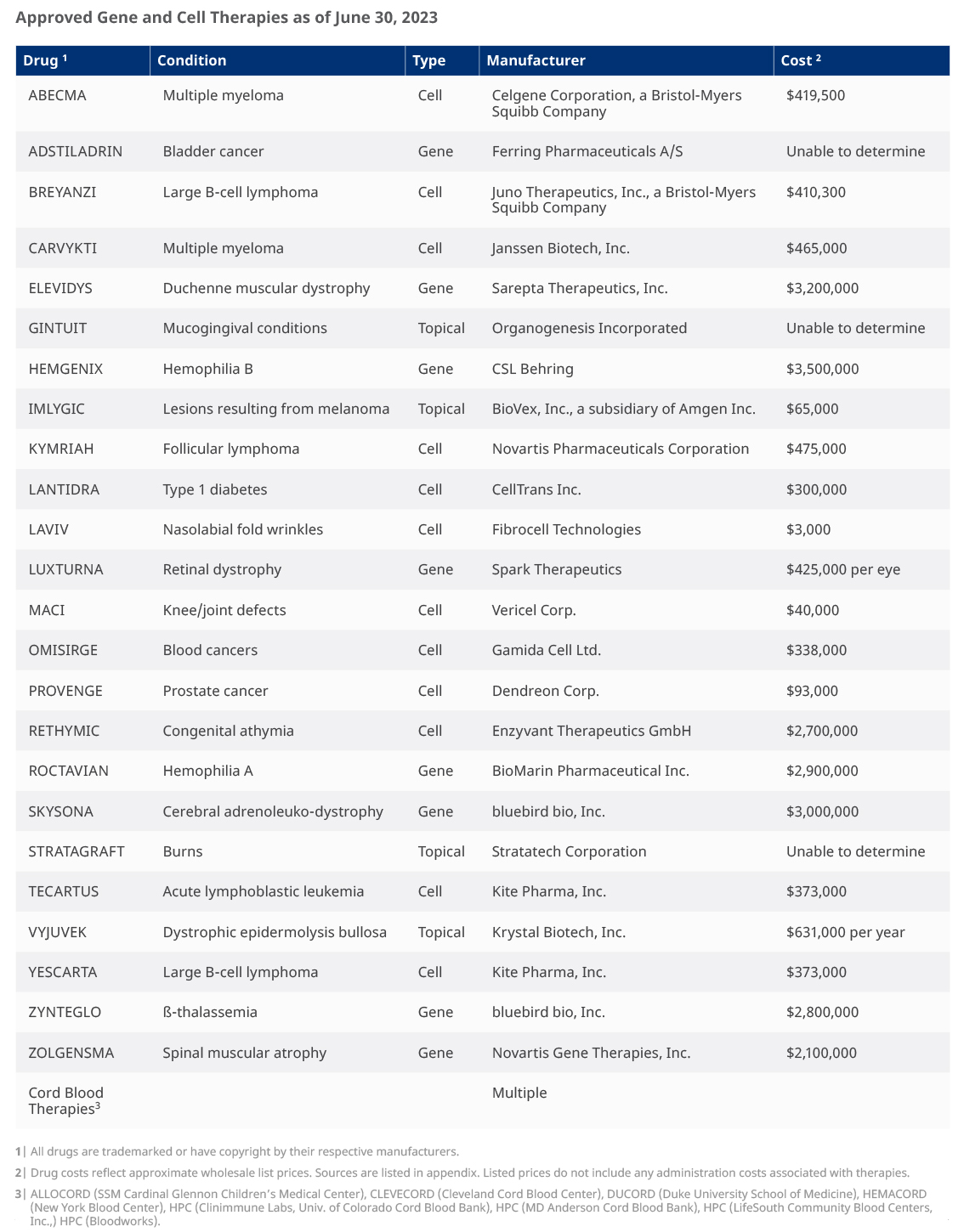
So far in 2023, the US Food and Drug Administration (FDA) has granted approval to 2 gene therapies and 1 cell therapy.
- Elevidys, an in-vivo gene therapy manufactured by Sarepta Therapeutics, was approved on June 22 to treat pediatric patients with Duchenne muscular dystrophy, which affects approximately 1 in every 3,500 male births.1 Analysts predict Elevidys treatment to cost USD 3.2 million.
- Lantidra, by CellTrans, was approved a week later on June 28. It treats adult patients with Type 1 diabetes via allogenic (donor) cell therapy. Approximately 1.6 million adults over the age of 20 have Type 1 diabetes.2 Lantidra is expected to cost approximately USD 300,000 per patient.
- The most recent FDA approval was granted to BioMarin Pharmaceuticals on June 29 for the in-vivo gene therapy Roctavian. Roctavian, a widely anticipated drug, aims to treat adult patients with severe Hemophilia A, at a price tag of approximately USD 2.9 million. Hemophilia A affects mostly males in the US—the US Centers for Disease Control and Prevention (CDC) suggests about 400 babies are born with the disease each year, with severe patients showing symptoms within one month of birth.3
These 3 therapies approved in the first half of 2023 bring the total count of approved gene and cell therapies to over 30. See below for a list of the FDA-approved therapies as of June 30, 2023.
As the number of approved gene and cell therapies continues to grow, the impact on insurance claim activity is a paramount challenge for employers, insurers and reinsurers. The eligible population to receive these therapies is still relatively small, so while currently approved therapies have not yet become a significant percentage of overall US medical spend, claim frequency is slowly building.4
At this point, we have seen a limited number of claims flow up to reinsurance, the majority of which have been claims for the Zolgensma therapy to treat spinal muscular atrophy. On average, the claims that we’ve seen for this therapy total approximately USD 2.19 million per patient.5 In addition to the lofty price tags of the therapies themselves, employers and payers are also confronted by the additional and substantial costs of administering the drugs, including hospital inpatient stays, chemotherapy, blood transfusions and follow-up visits.
- rarediseases.org/rare-diseases/duchenne-muscular-dystrophy/
- cdc.gov/diabetes/data/statistics-report/diagnosed-diabetes.html
- cdc.gov/ncbddd/hemophilia/data.html#:~:text=In%20the%20United%20States&text=Hemophilia%20A%20affects%201%20in,with%20 hemophilia%20A%20each%20year
- sunlife.showpad.com/share/qn4HBwhB34l6lpfgmz2Qd
- Claim totals inclusive of drug and related administration
Gene and Cell Therapies: Navigating the Challenges Around Rising Costs in Medical Insurance
The best way to pay for gene and cell therapy claims is still up for discussion. Given the FDA pipeline of therapies targeting much more common medical conditions, we expect claim volume and severity to increase in coming years. It is estimated that more than 1 million individuals will be eligible for gene and cell therapy treatment within 12 years.
One recurring risk transfer consideration is to carve out coverage of these therapies similar to the widely utilized organ transplant carve-out products. A handful of third-party entities (brokers, insurers and service providers) have introduced carve-out solutions over the past couple of years. However, there is a vast disconnect between what these solutions cost and what insurers are willing to pay for them. Brokers have yet to see any large uptake of carve-out solutions at this point. Instead, gene and cell therapy claims are widely covered under traditional reinsurance agreements, allowing some risk transfer to occur and reduce the financial strain of high-cost claims. As we look ahead, the question is how long traditional reinsurance mechanisms will be able to provide coverage for the expanding list of approved high-cost therapies.
Overall, the topic of gene and cell therapy remains a highly discussed yet relatively unaddressed problem.
Looking Ahead
As we pass the halfway mark of the year, 3 drugs remain in the pipeline for 2023, with Prescription Drug User Fee Act (PDUFA) dates in November and December 2023. PDUFA dates represent the date by which the FDA will either approve the new therapies or request further information from manufacturers. Notable therapies awaiting approval in 2023 include an ex-vivo gene therapy targeting sickle cell disease, a tumor-infiltrating cell therapy targeting metastatic melanoma, and an expansion of an existing cell therapy targeting multiple myeloma.
In 2024, we expect the FDA to consider therapies for blood disorders (such as transfusion-dependent beta-thalassemia and fanconi anemia), cancerous diseases (such as cervical cancers, Epstein-Barr virus-associated post-transplant lymphoproliferative diseases, marginal zone lymphomas, myxoid/round cell liposarcoma, synovial sarcomas), skin disorders (recessive dystrophic epidermolysis bullosas), immunodeficiencies (leukocyte adhesion deficiencies), metabolic disorders (metachromatic leukodystrophy, mucopolysaccharidosis), retinal diseases (leberhereditary optic neuropathy, X-linked retinitis pigmentosa, wet age-related macular degeneration), and neurodegenerative disorders (aromatic L-amino acid decarboxylase deficiencies and amyotrophic lateral sclerosis).6
As the coming years bring new therapy approvals, equally significant will be the impact of drugs that entered the market in 2022 and 2023. As eligible patients begin their treatments with these new therapies, insurance markets will begin to receive and react to more claims. With regard to gene therapy specifically, the industry is predicting that the cost for the 5 in-market gene therapies plus the 8 pending therapies in 2024 will cost $2.88 per member per month (PMPM) and $5.08 PMPM for commercial and Medicaid books, respectively.
Over the past 6 months, key players have engaged in continued discussion about new products and solutions to address this issue. Stop loss carriers and reinsurers are still considering carve-out products, aggregating deductibles, risk pooling and other financial risk transfer mechanisms. However, the cost of these solutions continues to outweigh the desire to buy, and most insurers have yet to pull the trigger on a reinsurance solution specifically addressing gene and cell therapy. As more therapies receive approval and claim frequency grows, we anticipate more uptake on varying types of capital protection, particularly in the small to mid-market sector.
How Guy Carpenter Can Guide You
Guy Carpenter is your partner in navigating exposure to the gene and cell therapy market. We assist our clients in understanding the landscape of gene and cell therapy, and identifying risk transfer solutions through traditional, carve-out or other strategic reinsurance arrangements.
We also know that financial risk transfer is not the only important topic within gene and cell therapy, which is why we also help our clients identify the right partnerships in the space for cost and care management. By introducing our clients to a third-party service provider, we help you finance, manage, and prepare for this evolving risk with the right expertise. Vetted third-party providers can provide clients with value-based care solutions, including pay-for-performance (warranty) contracts, and introduce clients to marketplaces for payer/employer/pharmaceutical interaction, which improves communication along the reimbursement pathway.
Guy Carpenter along with third-party providers can also assist in delivering useful predictive analytics, including ground-up PMPM costs by population, pharmacogenomics and rate making/pricing support. Alongside this, pipeline analyses determining estimated utilization, prevalence and cost metrics by therapy are vital to evaluating exposure.
Further, care management and cost management will be crucial tools in managing overall spend on these therapies. Payers must be able to track patient outcomes and clinical data, evaluate appropriateness, safety and efficacy of therapies, including prior authorization review, and must review orphan drug bills and conduct claim audits. Lastly, it is imperative that insurers are aware of how their centers of excellence and network development, formulary and plan benefits, and contract negotiations can impact how a gene or cell therapy claim will affect them. Guy Carpenter can help you ensure you have the proper management mechanisms in place.
Please contact your Guy Carpenter representative with questions or to discuss further.
Contacts
Ryan Keith |
Charlotte Stabler |
| Senior Vice President | Assistant Vice President |
| Charlotte.Stabler@guycarp.com |